Packing in voids of ionic solids
Packing in voids of ionic solids: Overview
This topic covers concepts, such as, Rock Salt Structure,Zinc Blende Structure,Radius Ratio etc.
Important Questions on Packing in voids of ionic solids
A compound AB has rock salt type structure. The formula weight of AB is 6.023 Y amu, and the closest AB distance is nm, where Y is an arbitrary number. The density of lattice is
In which of the following crystals, the alternate tetrahedral voids are occupied?
The edge length of ( structure) is . Assuming contact along the cell edge, calculate the radius of ion
An ionic crystal lattice has radius ratio of . Its coordination number is
Explain how to deduce coordination number of cations.
Write note on radius ratio rule for ionic compounds.
The distance between and in crystal is more than half of edge length.
The number of triangular voids in the given arrangement in the enclosed region is .
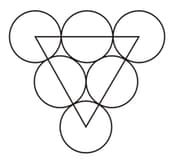
In structure, and ions are present in each unit cell.
In a solid, ions are packed in lattice. occupy half of the tetrahedral voids in an alternating arrangement. Now if a plane is cut (as shown) then the cross-section would be:
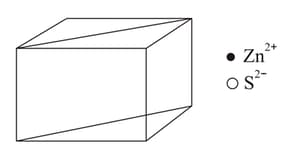
In the sphalerite structure, ions form a face-centred cubic lattice. Then ions are present on the body diagonals at a distance of __________ from the corner.
The range of radius ratio for an octahedral arrangement of ions in an ionic solid is
crystallises in the same type of lattice as does . Given that, and .
Calculate the ratio of the side of the unit cell of to that of .
An ionic compound is expected to have a tetrahedral structure if lies in the range of
How many unit cells are present in a cube-shaped ideal crystal of of mass ? (Atomic masses: )
A binary solid has a rock salt structure. If the edge length is and the radius of the cation is then, the radius of the anion is
In a sodium chloride structure, the percentage of the octahedral voids occupied by cations is
In the crystal lattice of diamond, carbon atoms adopt
In zinc blende structure, the coordination number of ion is
A solid has type of structure. If the radius of the cation is , then the radius of the anion in using octahedral dimension will be
In sodium chloride, ions form arrangement. Which site will ions occupy in this structure?
Which one of the following statements is incorrect about rock salt type?
